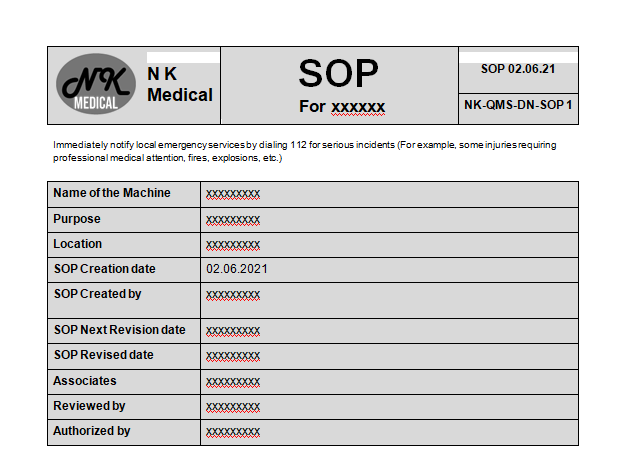
Standard Operating Procedures (SOP)
Standard Operating Procedures (SOP) are essential documents that detail the steps required to perform specific tasks or operations consistently and accurately. Widely used in industries such as manufacturing, healthcare, and pharmaceuticals, SOPs ensure uniformity, compliance, and quality in processes.
What is an SOP?
An SOP is a written document that provides clear, detailed instructions on how to carry out a particular operation or task. Its goal is to ensure that each person performing the task follows the same steps in the same order, achieving the desired outcome every time.
Key Components of an SOP
- Title and Purpose
- Title: Clearly state the name of the procedure.
- Purpose: Explain the necessity of the SOP and its intended outcome.
- Scope
- Define the boundaries of the SOP, specifying which activities or processes are included and which are not.
- Responsibilities
- Identify who is responsible for performing the procedure.
- Include any specific qualifications, training, or certifications required.
- Procedure
- Provide a detailed, step-by-step description of the procedure.
- Use clear, concise language, and include diagrams, flowcharts, or other visual aids if necessary.
- Safety and Compliance
- Outline any safety precautions or personal protective equipment (PPE) required.
- Reference relevant regulatory or compliance standards that must be adhered to.
- Documentation and Records
- Specify any forms, logs, or records that need to be completed as part of the procedure.
- Provide instructions on how to document and store these records properly.
- Review and Revision History
- Document the history of reviews and revisions to the SOP.
- Include dates, a brief description of changes made, and the names of reviewers.
Importance of SOPs
- Consistency and Quality: Ensure that tasks are performed consistently and correctly, reducing variability and enhancing quality.
- Training and Onboarding: Serve as essential training tools for new employees, helping them understand and perform their duties effectively.
- Compliance: Help organizations comply with industry standards and regulatory requirements, reducing the risk of legal issues and fines.
- Efficiency: Streamline operations by providing clear instructions, reducing downtime, and minimizing errors.
- Safety: Enhance workplace safety by outlining necessary precautions and ensuring tasks are performed safely.
Creating and Maintaining SOPs
- Develop a Standard Format
- Use a consistent format for all SOPs to make them easy to understand and follow.
- Involve Subject Matter Experts
- Collaborate with employees who regularly perform the tasks to ensure accuracy and practicality.
- Drafting the SOP
- Write the procedure in clear, simple language.
- Include detailed steps, necessary tools, and any safety considerations.
- Review and Approve
- Have the SOP reviewed by relevant stakeholders and subject matter experts.
- Make necessary revisions based on feedback before final approval.
- Implement and Train
- Distribute the SOP to all relevant personnel.
- Provide training on the new or updated SOP to ensure everyone understands and follows it.
- Regular Updates
- Review and update SOPs regularly to reflect changes in processes, technology, or regulations.
- Document any revisions and ensure the most current version is in use.
- Document Control
- Implement a document control system to manage SOPs.
- Ensure that only authorized individuals can make changes and that previous versions are archived.
By developing and maintaining comprehensive Standard Operating Procedures, organizations can achieve operational excellence, ensure compliance, and foster continuous improvement.

Reviews
There are no reviews yet.